
The Philippine Food and Drug Administration (FDA) has approved alpelisib in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor positive, human epidermal growth factor receptor-2 negative (HR+/HER2-), PIK3CA-mutated, advanced or metastatic breast cancer after disease progression following an endocrine-based regimen.1
Breast cancer is the most common cancer among Filipino women, and the third leading cause of cancer-related deaths in the country, next to lung and liver cancer.2
“Alpelisib was discovered at the Novartis Institutes for BioMedical Research. It is the first-ever treatment specifically for HR+/HER2- advanced breast cancer with a PIK3CA mutation. We are pleased to bring to the Philippines a new treatment option that specifically addresses the needs of the patients living with this mutation,” said Mr. Joel Chong, Country President, Novartis Healthcare Philippines, Inc.
“The Philippine FDA approval of alpelisib is a welcome development to advanced breast cancer patients in the country who have the PIK3CA mutation, offering them new hope for a longer life without progression,” said Maria Luisa A. Tiambeng, MD, Director, Cardinal Santos Medical Center Cancer Center.
“The regulatory approval of alpelisib is a game changer in the way we practice medicine in advanced breast cancer. For the first time, physicians can test for PIK3CA biomarkers and develop a treatment plan based on the genomic profile of a patient’s cancer,” said Dr. Arnold John B. Uson, President, Philippine Society of Medical Oncology (PSMO).
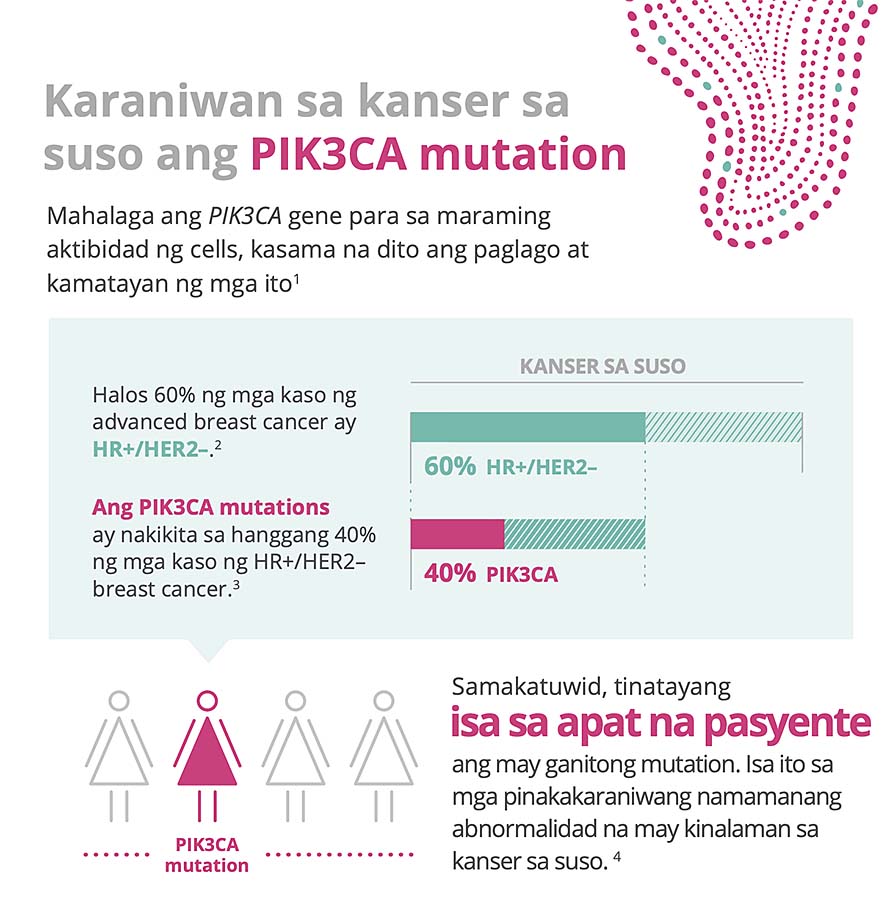
PIK3CA is the most commonly mutated gene in HR+/HER2- breast cancer; approximately 40% of patients living with HR+/HER2- breast cancer have this mutation. PIK3CA mutations are associated with tumor growth, resistance to endocrine treatment and a poor overall prognosis. Alpelisib targets the effect of PIK3CA mutations and may help overcome endocrine resistance in HR+ advanced breast cancer.
FDA approval is based on results of the Phase III trial, SOLAR-1, that showed alpelisib plus fulvestrant nearly doubled median progression-free survival (PFS) compared to fulvestrant alone in HR+/HER2- advanced breast cancer patients with a PIK3CA mutation. Alpelisib provided consistent PFS results across pre-specified subgroups, including among patients previously treated with a CDK4/6 inhibitor.
Overall response rate (ORR), an indicator of the proportion of patients who experience at least a 30% reduction in overall tumor size (in patients with measurable disease), was more than doubled when alpelisib was added to fulvestrant in patients with a PIK3CA mutation.
“We encourage patients with advanced breast cancer to undergo PIK3CA testing so that they can work with their medical team to create a customized treatment plan that can lead to enhanced treatment outcomes,” said Dr. Josephine Contreras-Tolentino, Chief, Section of Medical Oncology and Consultant Director of the Chemotherapy Unit of The Medical City.
For inquiries regarding the PIK3CA Testing Program, consult your oncologist. You may also contact Hi-Precision Diagnostics at (0908) 873-8370 / (0908) 880-2807 or email at [email protected]; or The Medical City at (02) 898-1000 local 3175 or 6415, (0961) 858-6606, or email at [email protected].
![]()







